Files
Download Full Text (660 KB)
Program
Internal Medicine
Training Level
Resident PGY 1
Institution
Henry Ford Hospital
Abstract
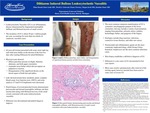
Diltiazem is a calcium ion cellular influx inhibitor approved by the U.S. Food and Drug Administration for the management of hypertension and chronic stable angina. Diltiazem is commonly used off label for chronic ventricular rate control in atrial fibrillation. Very few cases of widespread cutaneous vasculitis have been described in association with diltiazem since 1988. We report on a patient developing diffuse petechiae with overlying palpable purpura and tense bullae in both lower extremities, which progressed to the thighs, buttocks, abdomen, and upper extremities 6 days after starting diltiazem for management of atrial fibrillation. Skin biopsy revealed leukocytoclastic vasculitis.
Presentation Date
5-2020
Recommended Citation
Abou Asala, Elian D.; Gelovani, David J.; Clowney, Fiona; Scott, Megan; and Omar, Jasmine, "Diltiazem Induced Bullous Leukocytoclastic Vasculitis" (2020). Case Reports. 125.
https://scholarlycommons.henryford.com/merf2020caserpt/125